Acyclic and Cyclic Isoprenoids
Acyclic Isoprenoids
(shown above are molecular fossils, i.e. no double bonds) are just single isoprenoids concatenated to form a long chain. They may be connected in a regular head-to-tail manner, or an irregular tail-to-tail or head-to-head. They may also have cyclopentane and/or cyclohexane rings on the chains.
Some examples
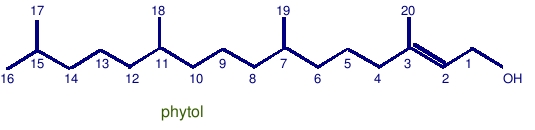
Phytol: C20 with a hydroxyl group and a double bond between the 2 and 3 carbon.
Phytol is derived from phytyl, the major side chain off of the chlorophyll-a molecule. When phytol undergoes diagenesis and catagenesis, pristane and phytane are two of the major biomarkers that are produced, though most regular isoprenoid alkanes containing 25 or less carbon atoms appear to originate from phytol.
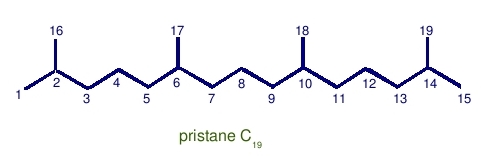
Pristane: C19 with head-to-tail linkage.
Can be derived by oxidation and decarboxylation of phytol.
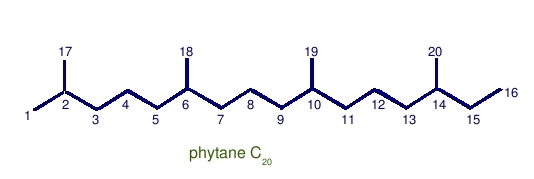
Phytane: C20 with head-to-tail linkage.
Can be derived from dehydration and reduction of phytol.
Notice that there is just a single methyl group difference between pristane and phytane. The nineteenth carbon atom in pristane is a methyl branch, not an additional carbon on the linear part of the chain.
The difference in pathways in the synthesis of pristane and phytane molecules make their ratios a biomarker for redox conditions at the time of burial (though it is not known as a very specific signal). In addition, they may be used as a signal of primary production, as they may be derived from the chlorophyll-a molecule.
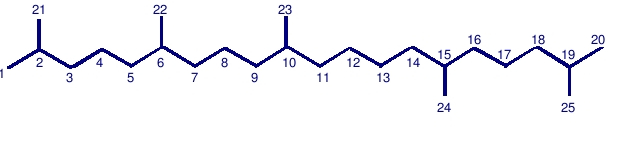
Pentamethyleicosane (PMI): C25 with tail-to-tail linkage.