Biomarker and How Molicular Fossil Formed?
This question might be asked by a medical student medical professional geological student or professional. Searching on the internet and writing “What is a Biomarker” will find many results but one is for the medical student and the other is for geological students namely all are the same because the biomarker is the end product from source organic matter.
The biomarker is the proxy for the paleoenvironment interpretation. The biomarker is named a biomarker that is related to the geological study. When an organic matter is discomposed, by some diagenetic processes, then some more stable organic matter is produced and they are called the biomarker or the geo-biomarker, the molecular fossils or geochemical fossils.
What is a biomarker and its application:
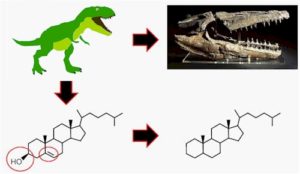
Biomarkers are generally derived from the lipids of organic matter. Suppose, Pristane, is a molecular fossil that indicates the redox environments and is widely recognized as a redox proxy.
How Biomarkers/molecular fossils are formed?
We can give an example of the production of Pristane in the sediments (Tetramethylepenta decane). Pristane is the branched acyclic isoprenoid containing 19 carbon atoms. A prominent peak of the Pristane elutes just after the C17 n-alkane peak in the Gas chromatography-mass spectrometry (GCMS) measurements.
The original source for the Pristane (Pr) is the phytol. Phytol is derived from Phytyle, a major chain off of the chlorophyll-a molecule from the tree leaf. If phytol undergoes diagenesis and categenesis then pristane and phytane (another related biomarker) are produced.
Pristane produces with the oxidation and by the decarboxylation of the phytol. The phytane has the carbon 20 atom, and is produced by the dehydration of the phytol and remain in the sediments as they are most stable. Some important biomarkers in the paleoenvironments study and petroleum industry.
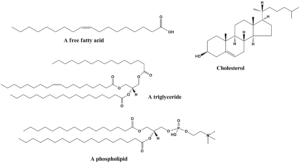
Moretane and hopane are the most important biomarkers in geochemistry for identifying the maturity of rocks and paleoenvironments of the past.
What is a biomarker and what is the list of important biomarkers:
Pristane (Pr), Phytane (Ph), Phenanthrene (Phe), Dibenzothiophene (DBF), Cadalane, Retane, DBF/Phe, Cadalane/Phe, Bonzo(e)pyrene, n-alkane, C33ACH (C33 n-alkane cyclohexane), Metane, Hopane, Sterane, Simonelite, coronene, Isorenairatane, 2-Methyl hopane, etc.
How do we know the soil erosion event occurred in the past?
The high value of the Pr/Ph ratio indicates the oxic condition in the soil or marine depositional environments. Hence, the d13 Corg positive shift indicates the relation between the soil erosion event and deposited sediments in the ocean bottom as the high value of the Pr/Ph in the ocean sediments indicates the oxic environments as for the incorporation of more soil from the land.
On the other hand, DBF/Phe high value also indicates the soil erosion event. So it must be seen the high value if there is a soil erosion event. The value of the Cadalane is also very important to make a correlation among them.
Because Cadalane is usually derived from the higher plants of terrestrial environments. Cadalane, retane, and Simonelite are all derived from higher plants, so, in the soil erosion events, the high value of Cadalane, retane, and Simonelite will be prominent in the rock samples. Another, evidence of the soil erosion event is the dominance of the higher n-alkane like, C25 n-alkane to C35 n-alkane.
Because these higher chain n-alkanes are usually derived from the higher plants or trees that contain higher cellulose. Hence, higher n-alkane is another proxy for the identification of soil erosion events. The total organic carbon from that sample will be high due to the incorporation of high organic matter during the soil erosion events.
Soil carries lots of organic matter along with the soil grain and is deposited in the ocean bottom. Hence, the TOC value will be higher in the rock samples. Soil erosion events may occur in many ways, like, huge volcanic eruptions, forest fires, meteorite impacts, etc, The main cause of the soil erosion event can be identified based on some important biomarker proxy, and among them, the Coronene index is one of them.

What is a Biomarker as a Coronene index can differentiate the forest fire and meteorite impact event
Coronene index indicates the event whether it was a forest fire, volcanic eruption, or massive meteorite impact based on the value of the Coronene index.
The coronene index=coronene/coronene+benzo (a) pyrene + benzo (g,h, i) pyrelene. This coronene index value increases if any extraterrestrial body hits the planet Earth where a higher amount of organic-rich sediment exists.
The combustion of coal or petroleum beneath the ground will produce a higher amount of coronene. A value of the coronene index higher than 0.8 indicates the meteorite impacts, less than 0.8-0.5 is for volcanic eruption and below 0.5 is for a forest fire event.
So, based on the observation of the coronene index value geologists can interpret paleo events like soil erosion events due to a forest fire volcanic eruption or meteorite impacts.
The evidence is the real fact because the coronene production in the rock sample depends on the heat used during the soil burning. If more than 4000 degrees Celcius is applied, a relatively higher amount of coronene will be produced, and if less heat is applied then less amount.
So, the coronene production in a rock sample is directly proportional to the heat applied. As the meteorite impact imposes huge heat in the soil hence high amount of coronene will be produced.
What is a biomarker?
Molecular biological markers, or biomarkers, are natural products that can be traced to a particular biological origin. They are powerful tools that can be used to trace diseases, drugs, and environmental contaminants in modern systems.
In our group, however, we use them to study ancient environments and the evolution of life on Earth. The most effective biomarkers/biomarkers are/are organic compounds with specific biological sources, whose structures can be preserved through geologic time.
Reconstructing early life from organic matter
Molecular fossils that are stable under geological conditions mostly originate from biological lipids. Lipids are a group of molecules that include fats and waxes, and they generally preserve better in sediments over long periods than molecules like DNA and proteins. These biomarkers encode information about ancient biodiversity, food chain associations, and environmental conditions.
They are recorders of element cycling, sediment and water chemistry, oxidation-reduction conditions, and temperature histories. Most importantly, however, hydrocarbon biomarkers are stable for billions of years if they are enclosed in intact sedimentary rocks that have only suffered a mild thermal history (i.e. haven’t been heated up very much).
Therefore, biomarkers offer a powerful means of studying geobiology—life and its interaction with the environment.
Structural and isotopic information allows them to be distinguished from abiogenic (non-living) organic compounds that exist throughout the cosmos. Thus, biomarkers are also an important tool in searching for extraterrestrial life elsewhere in the universe.
How biomarker is created and preserved in the geologic record
With that introduction, we can begin to get to the biological and geological processes that create and preserve biomarkers. Below is a geologic timescale from the beginning of the formation of the earth to the present day, which shows you what we have learned about the evolution of life through studying biomarkers.
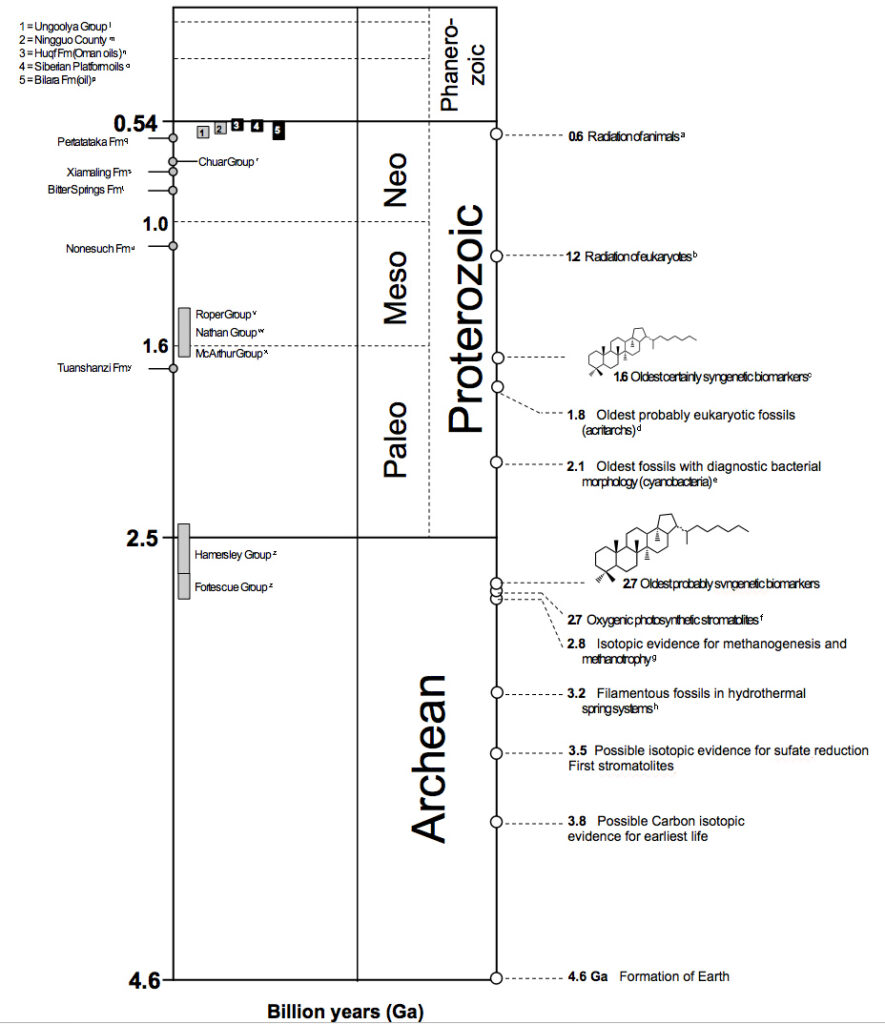
Biomarkers can be classified in this section read more to learn the types of biomarkers>>Biomarker classification
The lipids are the main source of all biomarkers hence it is generated from the lipids. Learn more from this section >>>Lipids
Some of the lipids are acetogenic lipids which are like alcohol, and cholesterol which do not dissolve in the water. Learn more from this section >>>>Acetogenic lipids
In this section learn more about the Isoprenoid. They are classified into
>>>>Acyclic and cyclic isoprenoids
Hence learn more about the >>>>Isoprenoids
In this section, you can learn more about hopanoids>>>>Hopanoids, hopanes
Learn more about the steroids. It is one of the important biomarkers in the geologic study>>>>Steroids, Steranes
Those biomarker has a larger Carbon number in the hopanoid ring hence the name carotinoid>>>>Carotenoids
>>>>Squalene
This is the source of all biomarkers>>>Kerogen
The process of forming biomarker >>>Diagenesis and catagenesis
Biomarkers can identify the source of any production of biomarkers. Learn more from this section >>Biomarker as Source Indicator
What is a biomarker and hydrocarbon? How it is linked with the biomarker or the molecular fossils? Learn more from this section >>>Hydrocarbons
>>Experimental methods to extract biomarkers
>>>Source rock
>>>Extraction
>>>GCMS
What are the Sources of Hopanoid
Hopanoid lipids are largely confined to eubacteria although the knowledge of their phylogenetic distribution is incomplete. Both Gram-positive and Gram-negative eubacteria produce hopanoids.
Hopanoids are relatively abundant in cyanobacteria, methanotrophic bacteria, and members of alpha-proteobacteria particularly the nitrogen-fixing eubacteria.
Hopanoids commonly occur in bacterial strains with high guanine and cytosine characteristics of species that occupy stressful ecological niches.
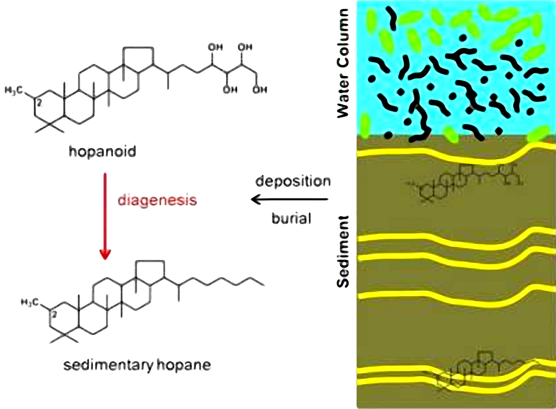
Sources of hopanoid and Biosynthesis
Their abundance may vary in response to osmotic stress (e.g. high salinity, sugar, or ethanol concentration) which increases the greater membrane stability.
Hopanoid biosynthesis does not require molecular oxygen, these compounds have not yet been discovered in obligate anaerobes.
Hopanoids have been detected in only approx. 30% of the bacterial species analyzed. The hopanoids also have not been detected in archaea or animals but small amounts occur in some ferns, mosses, and fungi.
Geologists Need to Know the Details: What are Coal Type Hopane?
What is Coal Type Hopane?
A high moretane/hopane ratio commonly occurs in the immature organic matter regardless of the lithology of sediments when accompanied by the high maturity stage, it usually indicates terrigenous plant-derived organic matter. This pattern of hopane distribution is called coal type hopane.
The high abundance of moretane is most probably related to acidic and oxic conditions in peat or coal-enriched sediments. Coal type Hopan is called because the ratio of Moretane and Hopan is much higher and this type of hopane anomaly is usual in the coal forming depositional setting in marine sediments, hence the name.
Interpretation of Coal Type Hopan and other related Biomarkers
Oxygen-containing aromatic compounds (such as dibenzofuran). These compounds represent the land-derived diagenetic products of polysaccharides and may be indicative of massive soil erosion (Sephton et al., 2005).
The C31 hopanes can be formed by the decarboxylation of the C32 hopanoid acids. The C32 Hopanoid acids are formed in the peat deposition environment or Oxic environments and during diagenesis, it becomes the C31 Hopane.
Very high Tm/C30 hopane and Tm/Ts are found in coals or coaly shales both in the immature and mature stages. Usually Tm (17a(H)-28,29,30-tris-norhopane) is less stable than Ts (18a(H)-28,29,30-tris-norneohopane).
If we found High Tm than Ts even in the mature stage it indicates the source input might be peat-forming processes because acidic clay may be the source of Tm and peat are the source of acidic clay.
The hopane/sterane ratio (Sum of C27–C35 hopanes/ Sum of C27–C29 steranes) is usually used to indicate the input of bacteria versus algae (phytoplankton) in sediments. High hopane/sterane ratios usually occur in terrestrial sediments and rocks where bacteria are dominant.
Unveiling the Marvels of Coronene: A Story of Molecular Beauty
Exploring the Wonders
Coronene, a captivating molecule with a mesmerizing structure, has captured the attention of scientists and enthusiasts alike. In this article, we embark on a journey through the realm of organic chemistry, uncovering the secrets of coronene and delving into its fascinating properties. Join us as we explore the molecular beauty of coronene, told through the lens of science and storytelling.
The Essence of Coronene: A Molecular Marvel
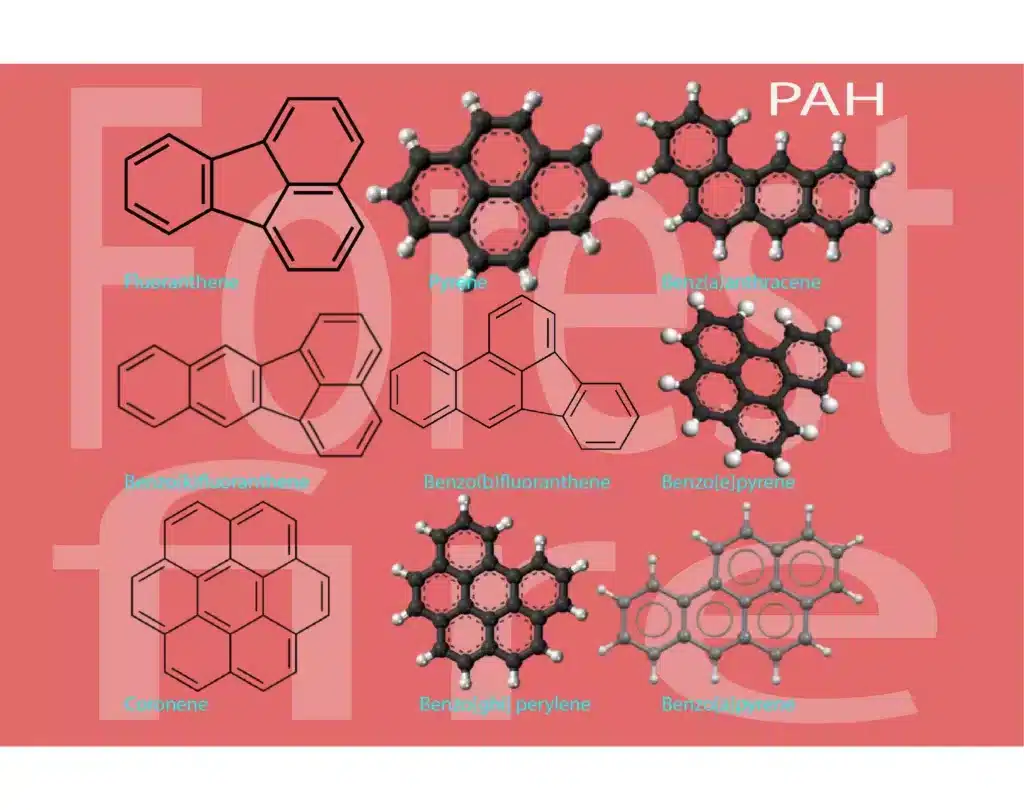
To truly grasp the marvels of coronene, we must first understand its composition and structure. Coronene belongs to a class of compounds called polycyclic aromatic hydrocarbons (PAHs), characterized by a series of fused benzene rings. With its distinct hexagonal shape and fused ring structure, coronene stands as a testament to the intricate nature of organic chemistry.
The Birth of a Molecule: A Stellar Nursery
Coronene is not a stranger to the cosmos. In fact, it is believed to form in the depths of interstellar space, where complex chemical processes give rise to its creation. Nebulas, the birthplaces of stars, are abundant with carbon-rich materials that serve as the building blocks for molecules like coronene. It is within these celestial nurseries that the story of Coronene begins.
The Structure of Coronene
The identification of Coronene (m/z 300) by (GCMS) Gas Chromatography mass spectrometry.
The identification of coronene based on a specific m/z value requires careful consideration and should be supported by additional evidence.
While m/z 300 can be a characteristic fragment ion associated with coronene, it is important to note that mass spectra can vary depending on instrument settings, ionization techniques, and other factors. Therefore, relying solely on a single m/z value for identification may not be sufficient.
To confirm the identification, it is advisable to compare the complete mass spectrum of the compound in question with reference spectra from authenticated coronene standards or reliable databases. Look for a combination of characteristic peaks and fragmentation patterns that align with the expected mass spectrum of coronene. Additionally, considering the retention time and employing other confirmation techniques, such as comparison with authentic standards or complementary spectroscopic methods, can further enhance confidence in the identification.
It’s crucial to approach the identification of coronene or any compound using GC-MS with a comprehensive analysis of the entire mass spectrum and considering multiple identification parameters to ensure accurate and reliable results.
The molecular structure is a sight to behold. Its hexagonal shape and fused benzene rings create a visually striking arrangement that sparks curiosity and wonder. Let us delve into the structural intricacies of coronene, examining its unique characteristics.
Hexagonal Symmetry
At the heart of coronene’s structure lies its hexagonal symmetry. This feature gives the molecule a sense of balance and harmony, as each benzene ring neatly connects to the next. The hexagonal arrangement not only adds to the aesthetic appeal but also contributes to its stability and physical properties.
Fused Ring System
Coronene’s fused ring system is another defining aspect of its structure. With each benzene ring bonded to its neighbors, the molecule forms a continuous, interconnected network. This fused ring system contributes to the molecule’s rigidity and creates a flat, planar structure, which plays a role in its unique electronic and optical properties.
The Quest for Understanding
Scientists have long been captivated by the properties and potential applications of coronene. Let us embark on a scientific quest, exploring the various aspects of this intriguing molecule and the discoveries it has unveiled.
Electronic and Optical Marvels
Coronene’s structure gives rise to a range of electronic and optical properties that have caught the attention of researchers. Its flat, planar structure facilitates efficient electron delocalization, making it an excellent candidate for electronic devices. Additionally, coronene exhibits remarkable light-absorbing capabilities, making it of interest in the field of optoelectronics and solar energy.
Aromatic Chemistry at Play
As a member of the PAH family, coronene falls within the realm of aromatic chemistry. Aromatic compounds, known for their distinctive and often pleasant odors, have widespread applications in perfumery, pharmaceuticals, and materials science. Coronene’s aromatic nature opens up avenues for innovative applications, making it a subject of interest for researchers across diverse disciplines.
Insights from Nature
While coronene’s origins lie in the vast expanse of the cosmos, its impact can be observed on Earth as well. In nature, coronene can be found in various forms, including as a component of fossil fuels and as a byproduct of combustion processes. By studying the behavior and transformations of coronene in natural environments, scientists gain valuable insights into the Earth’s carbon cycle and environmental processes.
Unlocking the Potential
Coronene’s remarkable properties and versatile nature have sparked excitement among scientists and innovators, who are unlocking its potential in a multitude of applications. From high-performance materials to advanced electronics, coronene’s unique attributes offer a world of possibilities waiting to be explored.
Advanced Materials
Coronene’s planar structure and electron-delocalizing properties make it a promising candidate for advanced materials. Researchers are exploring its incorporation into nanomaterials, polymers, and carbon-based composites, aiming to enhance their mechanical, electrical, and thermal properties. These advancements have the potential to revolutionize fields such as aerospace, electronics, and energy storage.
Environmental Implications
Understanding the behavior and fate of coronene in the environment has crucial implications for addressing pollution and environmental challenges. Researchers are investigating the interactions between PAH and environmental matrices, shedding light on its potential role in pollution mitigation and environmental remediation strategies.
A Molecular Tale
Coronene, with its hexagonal symmetry and fused ring structure, stands as a testament to the marvels of organic chemistry. From its origins in the cosmos to its potential applications on Earth, this molecule has left an indelible mark on the scientific community. The tale of coronene serves as a reminder of the intricate beauty that exists within the world of molecules and the endless possibilities they hold.
Why is Coronene Aromatic?
Coronene is considered aromatic due to its specific structural features and electronic properties. It belongs to the class of polycyclic aromatic hydrocarbons (PAHs) that exhibit aromaticity. Aromatic compounds, including coronene, possess a planar ring structure and a high degree of electron delocalization. In coronene, the fused benzene rings create a continuous, conjugated system of π-electrons, which results in exceptional stability and unique chemical reactivity.
Another Name for Coronene
Coronene is sometimes referred to by another name—superbenzene. This name stems from the fact that coronene consists of six fused benzene rings, which can be seen as an extension of the benzene molecule. The term “superbenzene” highlights the larger size and increased complexity of coronene compared to a single benzene ring.
Structure of Coronene
Already explained. Coronene possesses a distinct structure that contributes to its aromatic and unique properties. It consists of six benzene rings fused together in a planar, hexagonal arrangement. Each benzene ring shares carbon atoms with adjacent rings, forming a continuous network of sp2 hybridized carbon atoms. The resulting structure is flat, and rigid, and exhibits hexagonal symmetry, which contributes to its stability and aromatic character.
Structure of Pyrene
Pyrene is another polycyclic aromatic hydrocarbon (PAH) with a structure similar to coronene. It consists of four fused benzene rings arranged in a linear, non-planar structure. The four benzene rings in pyrene are connected in a “U” shape, resulting in a three-dimensional arrangement. Pyrene is often found as a component of fossil fuels, and it is also a common model compound in the study of PAHs due to its relatively simple structure compared to larger PAHs like coronene.
Unveiling the Significance of Polycyclic Aromatic Hydrocarbons (PAHs)
Polycyclic aromatic hydrocarbons (PAHs) represent a class of organic compounds that hold great significance in the field of chemistry. These compounds are characterized by the presence of multiple fused aromatic rings, giving rise to their distinct properties and applications. Let us delve into the world of PAHs, exploring their diverse aspects and understanding their importance.
PAHs: The Building Blocks of Organic Chemistry
PAHs serve as fundamental building blocks in the realm of organic chemistry. With their intricate structures and diverse properties, they lay the foundation for the synthesis of various organic compounds. PAHs are known for their stability and versatility, making them essential components in the development of pharmaceuticals, dyes, polymers, and other valuable products.
Superbenzene: Unraveling the Marvelous Synonym for Coronene
Superbenzene, a synonym for coronene, showcases the awe-inspiring nature of this remarkable compound. Comprising six fused benzene rings, superbenzene surpasses the simplicity of individual benzene molecules. Its larger size and increased complexity contribute to its unique chemical reactivity and captivating properties, captivating the attention of researchers and enthusiasts alike.
Fused Benzene Rings: Exploring the Intricate Architecture
The presence of fused benzene rings is a defining characteristic of PAHs. These rings form a continuous network, resulting in a complex and interconnected structure. The fusion of multiple benzene rings imparts distinct physical and chemical properties to PAHs, including their aromaticity, stability, and ability to engage in various chemical reactions.
Aromatic Hydrocarbons: The Essence of Distinctive Chemistry
PAHs belong to the class of aromatic hydrocarbons, known for their captivating aromatic character. Their structures possess conjugated systems of π-electrons, enabling electron delocalization and imparting unique stability. Aromatic hydrocarbons exhibit fascinating electronic properties, making them vital in the development of electronics, materials science, and other fields.
Hexagonal Hydrocarbons: Unveiling the Geometric Wonders
Hexagonal hydrocarbons encompass the intriguing world of PAHs. The hexagonal shape arises from the arrangement of benzene rings, forming a visually striking geometric pattern. This hexagonal symmetry imparts stability and aesthetic appeal to PAHs, serving as a testament to the geometric wonders that nature presents in the realm of organic compounds.
Coronene: The Molecular Marvel and Planar Hydrocarbon
Coronene, a prominent member of the PAH family, emerges as a molecular marvel and a prime example of a planar hydrocarbon. Its flat, planar structure ensures efficient electron delocalization, leading to its exceptional stability and aromatic character. Coronene represents the elegance and beauty that planar hydrocarbons exhibit within the vast realm of organic chemistry.
Aromatic Compounds: Embracing the Essence of Distinct Aroma
Aromatic compounds, including PAHs, epitomize the essence of distinct aroma. The term “aromatic” originally referred to their strong and pleasant odors. However, in the context of organic chemistry, aromaticity signifies a specific electronic and structural phenomenon. Aromatic compounds exhibit unique stability, reactivity, and resonance, making them pivotal in diverse scientific disciplines.
Hexagonal Compounds: Exploring the World of Geometric Marvels
Hexagonal compounds, like PAHs, captivate scientists and researchers with their intriguing geometric nature. The hexagonal shape, resulting from fused benzene rings, creates visually appealing structures with inherent stability and symmetry. Hexagonal compounds showcase the interplay between molecular architecture and geometric marvels within the realm of organic chemistry.
